LifeTech Scientific Corporation
LifeTech Announces 2015 Annual Results
******************
Global Channel Expansion, Stable Growth of Domestic Market Sales,
Diversified Product Portfolio and Innovation Promote Sustainable Development
Steady Growth of Revenue and Significant Rise of Profitability
Financial Highlights
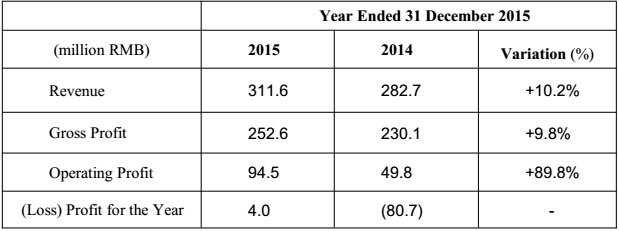
Main Achievements
The performance is continuing growing: the turnover reached RMB 311.6 million, with year-on-year growth of 10.2%. The domestic sales witnessed a steady growth of appropriately 21.2% compared with the same period of 2014, further strengthening the market position. Operating profit was RMB 94.5 million, with year-on-year growth of 89.8%. Profit for the year was RMB 4.0 million, with the listing and option costs, Medtronic consulting fees, effect on convertible bonds, and non-recurring profit and loss excluded, the figure was RMB 94.4 million, representing a growth of 40.5% compared with the same period last year(2014: RMB 67.1 million).
Remarkable achievements made in R&D: with the philosophy of producing innovative products, LifeTech Scientific Corporation had made significant achievements in the new product research and development in the Report Period:
1. “New Equipment, New Technology and Clinical Application of Ventricular Septal Defect Intervention Therapy” won the second prize of National Award for Technological Invention in 2014.
2. LAmbreTM LAA occluder: completed the clinical trial in Europe and twelve-month follow-up visit in China; expected to get CE certification in 2016
3. Absnow™ absorbable occluder system was awarded the Silver Award of 2015 China Red Star Design Award.
4. GoldenFlow™ peripheral stents: CE certification acquired
5. Ankura II TAA stent graft system: registration application had been submitted for CFDA
6. Clinical trials for several new products were carried out smoothly.
At the same time, to accelerate the development of new product and dedicate to international academic communication and clinical training, LifeTech Scientific Corporation had completed the construction of South China’s first high-end animal laboratory in Shenzhen.
Continued growth of current products: benefited from continuous selection of quality distributors and the establishment of a large number of distribution lines, the increase of professional products promotion activities, the expansion of sales coverage, the adoption of new sales policy and the great control of sales focus, the sales of main products of LifeTech had achieved a continued growth in the report period. In which, the sales of peripheral vascular diseases products witnessed a significant growth, reaching 16.2%.
Expansion of global channel: during the reporting period, LifeTech kept consolidating its current businesses of main products while actively dedicating to expanding the distribution network in foreign and domestic markets. As of December 31, 2015, the LifeTech product distribution network covered 76 countries and areas around the world, and had 164 quality distributors.
Great strategic alliance with Medtronic: LifeTech established a smooth cooperation with strategic shareholder Medtronic during the reporting period. Medtronic was responsible for sole selling of the occluders, which independently developed by LifeTech, in western Europe and Middle East. At the same time, through introducing the world-class technology and quality system of Medtronic, LifeTech will produce and commercialize its branded pacemaker in China. The pacemaker clean workshop had been built in the report period. It is estimated that the pacemakers will be put into clinical trial and a registration application will be submitted to the CFDA for approval in 2016.
March 29, 2016, Hong Kong - the world’s leading supplier of minimally invasive interventional medical devices designed for curing cardiovascular and peripheral vascular diseases ("LifeTech" or "The Company", HKEX stock code: 1302) and its affiliated companies ("The Group") issued its audited comprehensive performance as of December 31, 2015 (within the reporting period).
The Group had achieved a steady growth during the reporting period: the turnover reached RMB 311.6 million, with year-on-year growth of 10.2% (the same period in 2014: RMB 282.7 million). The operating profit was RMB 94.5 million, with year-on-year growth of 89.8% (the same period in 2014: RMB 49.8 million). Profit for the year was RMB 4.0 million, with the listing and option costs, Medtronic consulting fees, effect on convertible bonds, and non-recurring profit and loss excluded, the figure was RMB 94.4 million, representing a growth of 40.5% compared with the same period last year (2014: RMB 67.1 million).
Mr. XIE Yuehui, the Chairman, CEO and Executive Director of LifeTech, expressed that: “faced with the uncertain global economic environment, continuous depreciation of worldwide currency against United States Dollar and the on-going market weakness, coupled with revolution of medical devices bidding process in China, our business is full of challenge during the year of 2015. We have withstood the challenge. The Group still made a growth under this uncertain situation, which is mainly due to its rich and diversified product portfolio, wide and high efficiency global sales network. It takes all feasible measures timely to maintain the continued growth of business and market share of the Group while facing multiple tests from outside.”
Remarkable achievements in R&D:
The Group had completed the construction of the Preclinical Cathlab in Shenzhen of the PRC. The Preclinical Cathlab is the first laboratory fully-equipped for animal experiment in the southern PRC and one of the high-level domestic animal experiment platforms in the PRC. The laboratory is also the only laboratory in the PRC which can be used for device-implantation experiments, pathology evaluation and pharmacokinetics testing, as well as physical and chemical testing, failure analysis, packaging, sterilization and regulatory consultation and preparation of registration documents at the same time.
The “New Equipment, New Technology and Clinical Application of Ventricular Septal Defect Intervention Therapy” developed and invented by Lifetech team with a help of clinical experts for more than a decade won the second prize of National Award for Technological Invention in 2014. In the same year, Absnow™ absorbable occluder system was awarded the Silver Prize of the China Red Star Design Award. Absnow™ absorbable occluder system is made by biological absorbable materials, realizing ideal treatment effects. At present, it is in the process of preclinical preparation. The launch of such product not only brings about the complete revolution in the field of interventional treatment for congenital heart disease, but also raises China’s medical technology level.
We had completed the clinical trial in Europe and twelve-month follow-up visit in China for the LAmbreTM LAA occluder, and had submitted the registration application for CE approval in Europe. A registration application will also be submitted for China CFDA approval. At the beginning of 2014, LAmbreTM LAA occluder had been approved as the innovative medical device by CFDA, which may speed up its registration procedure in China.
In addition, clinical trial of Ankura II TAA stent graft system had been finished and a registration application had been submitted to CFDA. Apart from the above achievements, the Group had obtained 130 product certifications in several countries and areas as of December 31, 2015, producing great chance for entering more international markets in the next few years.
Enormous work and effort had been done by the Group on clinical trials of innovative products. On October 30, 2015, the Group had finished the first implanting of GoldenFlowTM into human body in Prince of Wales Hospital in Hong Kong. GoldenFlowTM was positioned accurately and released steadily, proving a high degree of safety. The clinical trial registry of GoldenFlowTM in China was approved by the ethic committee of some clinical centers and will start the first clinical implantation. It has been granted the CE certification in February 2016 as the clinical trial is exempted in Europe.
In addition, the clinical trial of the Group’s FIM aortic single branch stent graft system and Iliac branch stent graft system also went on smoothly. FemoFlow™ Drug Eluting Peripheral Balloon Catheter had got the ethic approval from Shanghai Zhongshan Hospital for first clinical implantation. Lung volume reduction bronchial valve is under development, the design has been changed from passive lung volume reduction into active model, and accordingly the name has been changed to volume reduction warrior circle. So far, the design has been finalized.
Continued growth of current products:
As of December 31, 2015, the turnover of the Group was appropriately RMB 311.6 million, an increase of RMB 28.9 million or about 10.2% compared with the same period of the last year. The increase of turnover was mainly due to the expansion of current product sales network, the increase of penetration of more hospitals, and the rise of China’s market share. The turnover contributed by the congenital heart diseases business was approximately RMB142.4 million, with a growth of 3.7%; the turnover contributed by the peripheral vascular diseases business was about RMB169.0 million, with a growth of 16.2%. The sales of vena cava filter and stent graft saw ideal growth of 13.1% and 18.5% respectively. In addition, turnover from surgical vascular repair business was RMB 275,000, a huge increase from the RMB 12,000 in 2014. This was resulted from the third supplemental agreement signed between the Company and Medtronic during the reporting period, which allows the Company to distribute the improved valve products directly to its external customers.
Great strategic alliance with Medtronic:
The year of 2015 is another milestone for our strategic alliance with Medtronic via pacemaker project. Since October 2012, the Company or its affiliates had signed a series of strategic alliance agreements with Medtronic, Inc. or its affiliates ("Medtronic"), and expend the alliance to include making and commercializing pacemaker and cardiac lead products for China market in China in July 2014. The sterile cleanroom for pacemakers has been built, and the pacemakers will start clinical trial and apply for CFDA registration in 2016. At the same time, as the sole distributor of CeraFlexoccluders in specific countries in Europe and Middle East, Medtronic promoted the occluders independently developed by LifeTech through its powerful channel in Europe.
The strategic alliance will speed up the development of global brand and the increase of revenues of the Company. The expansion of a solid partnership will bring significant financial benefits, raise clinical competence, get extra training and education opportunities, and increase the brand awareness. The Company will further promote the alliance relation so as to increase the recognition of our brand in the world.
Expansion of global channel
The Group kept consolidating the current main products (occluders products, vena cava filter and stent graft) businesses and were active in expanding distribution network at home and abroad, increasing sales efforts and improving market penetration. As of December 31, 2015, the LifeTech product distribution network covered 76 countries and areas around the world, and had 164 quality distributors.
Prospects
Seen from current sustainable and fast development of innovative technologies in China market, the Group believes that the domestic sales will continue increasing. Also, by taking advantages of the competitive products and contributing more investments in global competition in overseas market, the Group is seeing a brighter prospect. Meanwhile, the partnership with Medtronic helps us integrate the advanced professional technologies to support our sustainable growth, development planning and the manufacturing ability, so as to provide better services for patients and doctors in China and around the world.
Mr. XIE Yuehui concluded that: with the owned advanced technologies and the ability to develop new patented products, the Group will continue to make breakthrough in R&D and commercialization of cardiovascular, peripheral vascular, cardiac pacing and electrophysiology and other implant products. By virtue of diversified owned innovative products, perfect product developing plan, powerful R&D strength, and wide global sales network, the Group is exploring new business growth points to produce bigger development space. Meanwhile, as a medical device enterprise with social responsibility, the Group will take the challenge and make progress based on strict internal control, high efficiency management and active attitude, offer quality services for global doctors and patients, provide ideal platform for employee’s development, and spare no effort to create more values for the partnership and shareholders.
###
About LifeTech Scientific Corporation:
LifeTech Scientific Corporation is the world`s leading supplier of minimally invasive interventional medical device for treatment of cardiovascular and peripheral vascular diseases and the Company is specialized in product R&D, manufacture and sales. Lifetech Scientific (Shenzhen) Co., Ltd. has been established in 1999, other subsidiaries and sales offices across the world have been set up afterwards. In November 2011, the Company was successfully listed in Hong Kong Stock Exchange. In October 2012, the Company reached strategic cooperation with Medtronic, the world top-notched medical device provider based in the US, and allowing Medtronic to become the strategic shareholder of the Company. As the world’s second largest (and the largest among BRIC countries) supplier of congenital heart occluder, Lifetech has significant influence on the global congenital heart occluder market. At present, the Company`s innovative products with self-owned intellectual property rights are well-marketed in 76 countries by more than 160 distributors. Meanwhile, the Company has undertaken various government research projects by virtue of its leading research capacity and facilities. The Company has also been granted the national high-tech enterprises and national post-doctoral research center in China.
(For more information, please visit /).
For any enquiry, please contact:
Ms. Gaby Yu Tel: +86 755 8602 6250 – 8916 Email: yujiahui@lifetechmed.com